Medical Transcription Training Center in Trivandrum
NATIONAL DRUG CODE
Section 510 of the Federal Food, Drug and Cosmetic Act (Act), 21 U.S.C. §360, requires a registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or processed by it for commercial distribution. Changes in the Act, resulting from the Food and Drug Administration Amendments Act of 2007 (Public Law 110-85) (FDAAA) require that drug establishment registration and drug listing information be submitted electronically unless a waiver is granted.
Drug products are identified and reported using a unique, three-segment number, called the National Drug Code (NDC), which is a universal product identifier for human drugs. FDA inputs the full NDC number and the information submitted as part of the listing process into a database known as the Drug Registration and Listing System (DRLS), which is transforming into the electronic system (eDRLS). The information submitted as part of the listing process, the NDC number, DRLS, eDRLS and the NDC Directory, are used in the implementation and enforcement of the Act.
The NDC, or National Drug Code, is a unique 10-digit or 11-digit, 3-segment number, and a universal product identifier for human drugs in the United States.
The 3 segments of the NDC identify: the labeler, the product, and the commercial package size.
- The first set of numbers in the NDC identifies the labeler, such as the DRUG MANUFACTURER, repackager, or distributer.
- The second set of numbers is the product code, which identifies the specific strength, dosage form (i.e, capsule, tablet, liquid) and formulation of a drug for a specific labeler.
- Finally, the third set is the package code, which identifies package sizes and types.
The 10-digit NDC will be in one of the following configurations: 4-4-2, 5-3-2, or 5-4-1, meaning that there are 4 or 5 digits for the labeler code, 4 or 3 digits for the product code and 2 or 1 digit(s) for the package code.
Example NDC
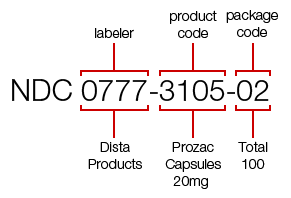
For example, the NDC for a 100-count bottle of Prozac 20 mg is 0777-3105-02.
The first segment identifies the labeler (the labeler code "0777" is for Dista Products Co., the labeler of Prozac).
The second segment, the product code, identifies the strength, dosage form (i.e, capsule, tablet, liquid) and formulation of a drug for a specific labeler ("3105" identifies that this dosage form is a capsule).
The third segment is the package code, and it identifies package sizes and types. The package code "02" for this bottle of Prozac identifies that 100 capsules are in the bottle.
Digital marketing, medical coding, medical scribing, medical transcription,

Comments
Post a Comment